Currently, lithium-ion batteries (LIBs) have achieved worldwide attention as advanced energy storage systems for commercial electronics and electric vehicles. Nevertheless, low abundance and uneven distribution of lithium resources have driven awareness that LIBs might not be able to fulfill the ever-increasing demand of grid-scale electrochemical energy storage systems. To spur more research into a more abundant and much cheaper candidate, researchers in China assessed proposed sodium-ion batteries (SIBs) and offered novel hard carbon anodes for more sustainable and cost-effective batteries.
They published their work on Mar. 10 in Energy Material Advances.
“Sodium-ion batteries (SIBs) have come under the spotlight for replacing lithium-ion batteries,” said paper author Ying Bai, professor at School of Materials Science and Engineering in Beijing Institute of Technology. “Thanks to cost effectiveness, inexhaustible Na resources, similar chemical nature of Na with Li, and similar operation mechanism to LIBs, the development of SIBs is highly desirable. Accordingly, it is critical to develop advanced electrode materials with excellent rate performance, good cycling stability and high-energy densities.”
Bai explained that among various anode materials, carbon materials may be the most likely candidates due to multiple superiorities, such as low cost, easy attainability and high cycling performance.
“Graphite represents the most promising carbon-based anodes that have been commercially used in LIBs for decades. However, graphite is not an appropriate choice for SIBs, because it is not energetically stable to form sodium-graphite intercalation compounds (Na-GICs),” Bai said. “Soft carbon anodes also show relatively lower sodium storage capacities be due to the insufficient interlayer distance for ion intercalation. Compared with soft carbon and graphite, hard carbons (HCs) possess relatively higher Na storage capacity because of the more heterogeneous structure that contains curved graphitic domains with large interlayer spacing for Na insertion and massive nanopores and edge terminations for Na adsorption.”
However, some obstacles hinder commercialization of HCs. According to Bai, the key challenges for HCs, especially their low ICE, poor cyclic stability, and poor rate capability, still demand deep exploration. Low ICE means existing nonreciprocal Na+ loss in side, requiring enough Na+ supply extracted from super-proportional cathodes when packing full batteries, which can decrease overall energy density and cycling performance of the full batteries. The poor rate capability limits their applications in high-power electronic devices, and poor cycling performance significantly hinders the practical realizations of SIBs. Therefore, advanced material engineering strategies are required for boosting SIB performances of HC anodes.
To enhance the Na storage performance of HCs, Bai said many studies focus on amorphous carbons with large specific surface area (SSA) or heteroatom-doped carbon materials with more defects, such as porous carbon, nanosized carbon, or heteroatom-doped carbon by anions. Bai and her team overviewed the advances of material engineering strategies for HC anodes.
First, nanostructure design provides controllable processing advantages in constructing carbons with hierarchical and complicated architectures, morphologies, and dimensionalities. Second, pore engineering with interconnected micro/meso/macropores can improve ion diffusion and the utilization of inner active sites. Third, defect engineering is effective to promote electrochemical activity of carbons to contribute high storage capacity.
Unfortunately, Bai said both large SSA and excessive defects in the carbon structure tend to induce uncontrollable decomposition of electrolyte and formation of uneven and unstable solid electrolyte interphase film (SEI), resulting in low ICE, poor cyclic stability, and decreased sodium diffusivity. Therefore, according to Bai, novel material optimization strategies are highly desirable.
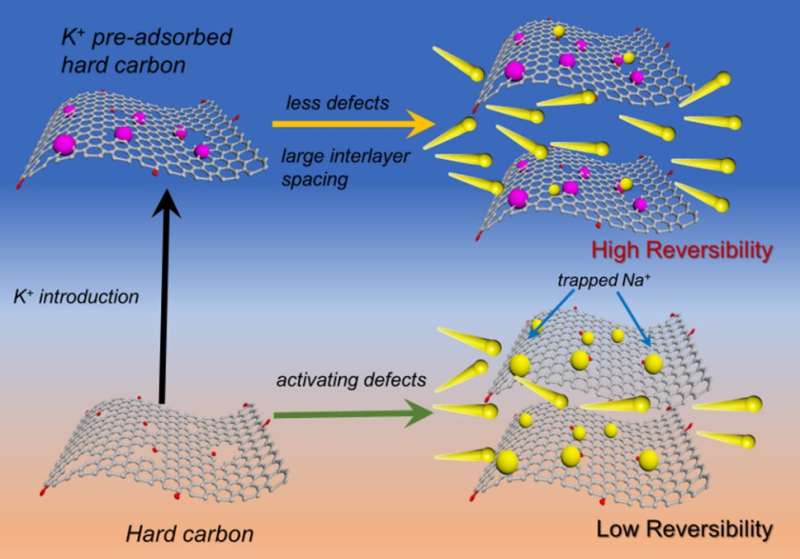
“Introducing cations can also regulate the microstructure of the HCs, such as interlayer spacing, electronic conductivity, graphite microdomains, and rebuild surface functionality, etc., while no extra active defects or pores formed. Therefore, cation-doping is imperative to optimize HCs with desirable physiochemical properties for high-performance anodes. In this work, we prepared K-doped HCs by annealing of K+ chemically-preabsorbed carbon resources. The K+ was selected to be preabsorbed on oxygen functional groups and some defects in HCs to deactivate these active sites, contributing to high ICE and high cycle stability.”
“Potassium has low ionization energy and can bind with negatively charged oxygen-containing functional groups with large electrostatic attraction, forming a stable structure,” Bai said. “The oxygen functional groups such as carbonyls and hydroxyls and some defect sites on carbon can work as anchoring sites for K+.” According to Bai, the K+ is chemically adsorbed on the oxygen functional groups by forming C-O-K bonds and occupying some defect sites.
“Therefore, irreversible adsorption of Na+ by oxygen functional groups and other defects can be reduced, thus leading to an improved ICE,” Bai said. “Meanwhile, the preabsorbed K+ can lead to carbon structural rearrangement during the carbonization process at high temperature, resulting in enlarged interlayer spacing and improved graphitization extent.” According to Bai, these structural evolutions lead to fast Na+ diffusion and higher conductivity, so that the rate capabilities of K+-preabsorbed hard carbon also get promoted, and a better ICE and an outstanding cycling stability can be also obtained.
This work put forward a novel, efficient, and low-cost way to improve the electrochemical performances of HCs. Bai said, such method is suitable for large-scale production, thus promoting the commercial application of HCs for SIBs.
“Although great achievements have been made, the development of practical HC anodes for SIBs is still facing massive challenges, such as complicated fabricating steps, complex microstructure of HCs, unclear Na storage mechanism, etc.,” Bai said. “Each type of carbon material has its own development bottlenecks, which are demand of advanced strategies to mitigate these issues. In addition, in-depth understanding of the electrochemical reaction mechanisms of carbon materials for SIBs is also equally important for high-performance material design. Overall, there is a long way to go for the commercialization of HCs and SIBs.”
Charged up: Revolutionizing rechargeable sodium-ion batteries with ‘doped’ carbon anodes
Ruiqi Dong et al, Tailoring Defects in Hard Carbon Anode towards Enhanced Na Storage Performance, Energy Material Advances (2022). DOI: 10.34133/2022/9896218
Provided by
Beijing Institute of Technology Press
Citation:
Tailoring defects in a hard carbon anode to enhance Na storage performance (2022, June 8)
retrieved 8 June 2022
from https://techxplore.com/news/2022-06-tailoring-defects-hard-carbon-anode.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
Stay connected with us on social media platform for instant update click here to join our Twitter, & Facebook
We are now on Telegram. Click here to join our channel (@TechiUpdate) and stay updated with the latest Technology headlines.
For all the latest Technology News Click Here
For the latest news and updates, follow us on Google News.
