A team of chemists at the University of Maryland has developed a next-generation aqueous electrolyte to reduce reliance on organic lithium-ion batteries. In their paper published in the journal Nature Energy, the group describes their approach to competing with commercial organic electrolytes. They demonstrate that LIB pouch cells can be made using aqueous electrolytes that have salt concentrations below 5 m. John Brown and Alexis Grimaud, with Collège de France and Electrochimique de l’Energie, respectively have published a News & Views piece in the same journal issue outlining the complications involved in developing replacements for organic lithium-ion batteries and the work done by the team in Maryland.
The work by the researchers involved searching for an aqueous electrolyte that went beyond water-in-salt, while also improving efficiency and stability. It had to have a salt concentration lower than 5.0 m (mol/kg) and a stability window greater than 3.0 V and reducing the cost to make it was important. They found a next-generation electrolyte that fulfilled all their requirements.
The work by the team involved mixing three eutectic materials together—CO(NH2)2, LiTFSI and water, along with the additive KOH, resulting in a 4.5m water-based electrolyte—a nonflammable ternary eutectic. They note that it is a low-cost solution that is also safe for the environment and opens the door to use of aqueous lithium-ion batteries being used in commercial products.
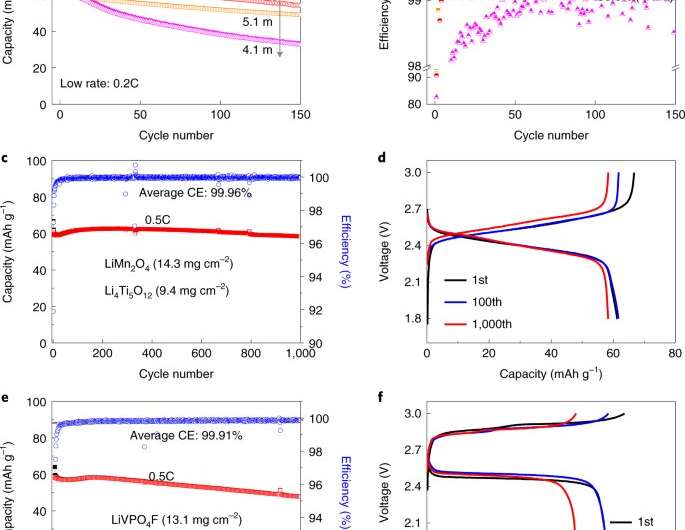
The team tested their electrolyte using Li4Ti5O12 as a negative electrode and LiMn2O4 as the positive electrode in a coin cell and found a CE of approximately 99.9%—a finding that suggests an increase of an order of magnitude in cycle life with coil cells. With pouch cells they found the electrolyte achieved 99.87% CE, also with an increase in cycle life. In testing discharge rate, they found it to be comparable to conventional pouch cells.
The researchers acknowledge that there is still work to do before their approach can be commercialized. First and foremost is the problem of decreases in water that occur during each cycle. To solve this problem, they suggest that the H2 gas that is generated during discharge be controlled.
Jijian Xu et al, Aqueous electrolyte design for super-stable 2.5 V LiMn2O4 || Li4Ti5O12 pouch cells, Nature Energy (2022). DOI: 10.1038/s41560-021-00977-5
© 2022 Science X Network
Citation:
A next-generation aqueous electrolyte (2022, March 15)
retrieved 15 March 2022
from https://techxplore.com/news/2022-03-next-generation-aqueous-electrolyte.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
Stay connected with us on social media platform for instant update click here to join our Twitter, & Facebook
We are now on Telegram. Click here to join our channel (@TechiUpdate) and stay updated with the latest Technology headlines.
For all the latest Technology News Click Here
For the latest news and updates, follow us on Google News.
